Rapid COVID-19 diagnostic test developed by Cambridge team to be deployed in hospitals
A new rapid diagnostic test for COVID-19, developed by a University of Cambridge spinout company and capable of diagnosing the infection in under 90 minutes, is being deployed at Cambridge hospitals, ahead of being launched in hospitals nationwide.
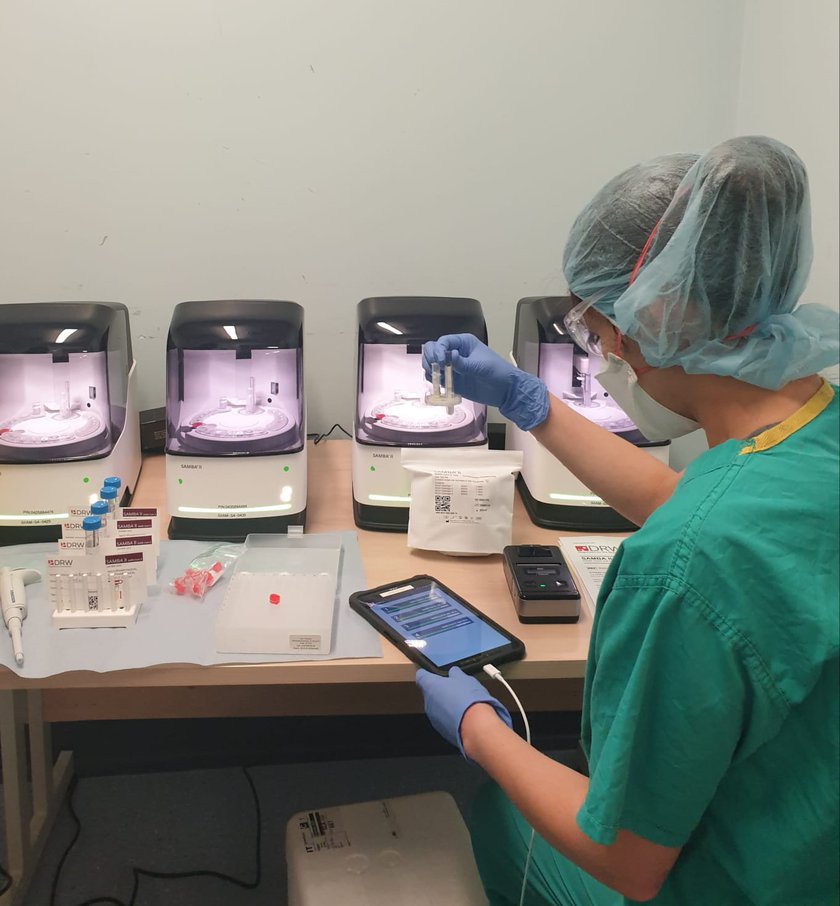
The SAMBA II machines, developed by Diagnostics for the Real World, provide a simple and accurate system for the diagnosis of COVID-19 infection. They will be used by healthcare workers at point-of-care in order to rapidly diagnose patients, directing those who test positive for the infection to dedicated wards. They can also help identify which healthcare workers are infected, enabling those who test negative to return to the front line.
The machines will be made available to a number of hospitals across the country thanks to a US$3million donation from the businessman and philanthropist Sir Chris Hohn, which will enable the purchase of 100 machines. The donation has enabled Addenbrooke’s Hospital, part of Cambridge University Hospitals NHS Foundation Trust, to obtain the first 10 SAMBA II machines this week for use in wards where suspected COVID-19 patients are brought in. The donation will be matched by the purchase of 10 additional machines by the Cambridge Trust.
SAMBA II looks for tiny traces of genetic material belonging to the virus, amplifies it billions of times chemically and is therefore extremely sensitive in the detection of active infections.
Patients will provide a nasal and throat swab. Once these have been loaded into the SAMBA machine, the remainder of the process is fully automated. At the moment, tests are sent for analysis in centralised laboratories and this, compounded by the sheer number of samples that are having to be analysed, means that diagnosis can take one to two days. SAMBA II is able to deliver results while the patient waits, helping healthcare workers ensure that those infected can be quickly directed to specialised wards. Whereas current tests can take over 24 hours or longer to deliver their results, SAMBA is able to deliver a diagnosis in less than 90 minutes.
The tests have been validated by Public Health England, Cambridge, in 102 patient samples and shown to have 98.7% sensitivity (ability to correctly identify positive cases) and 100% specificity (the ability to correctly identify negative cases) compared to the currently used NHS/Public Health England test. This has enabled the team to obtain a CE mark.
For more information visit: CUH News
Published April 2, 2020
Latest from CCTU
Targeting the immune system could prevent future heart attacks, Cambridge-led trial suggests
Cambridge researchers have discovered that an existing therapy which boosts protective immune cells in people who have recently had heart attacks reduces…
Cambridge study finds hot flush treatment has anti-breast cancer activity
A drug mimicking the hormone progesterone has anti-cancer activity when used together with conventional anti-oestrogen treatment for women with breast…
Innovative trial offers hope on World Pancreatic Cancer Day
An early-stage trial, recently opened at Addenbrooke's Hospital, offers new hope to people with late-stage pancreatic cancer and their families.
…






