COVID-19
Jump to TACTIC Clinical Trials
TACTIC
Multi-Arm Therapeutic Study in Pre-ICU Patients Admitted with COVID-19
There are currently no proven treatments to combat COVID-19 disease. Some people with COVID-19 develop severe symptoms which are thought to be the result of a heightened immune response leading to organ damage and, in some cases, death. The TACTIC programme is designed to assess selected medications which modify the immune response. These medications have been chosen by a consortium of clinicians and clinician-scientists with expertise in the treatment of immune-mediated disease and it is hoped that they will reduce severe symptoms and therefore the number of Intensive Care Unit (ICU) admissions in hospital.
The TACTIC programme uses a platform design which provides flexibility to swap out and add in alternative treatments depending on whether the data show:
- Effectiveness - in which case the treatment should be moved into NHS clinical care
- Ineffectiveness or safety concern - in which case the treatment should be withdrawn from the study
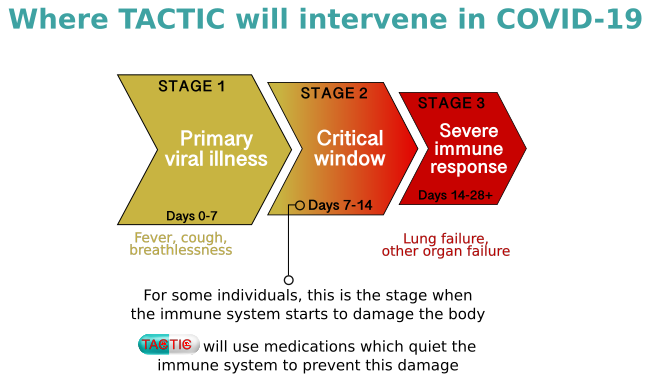
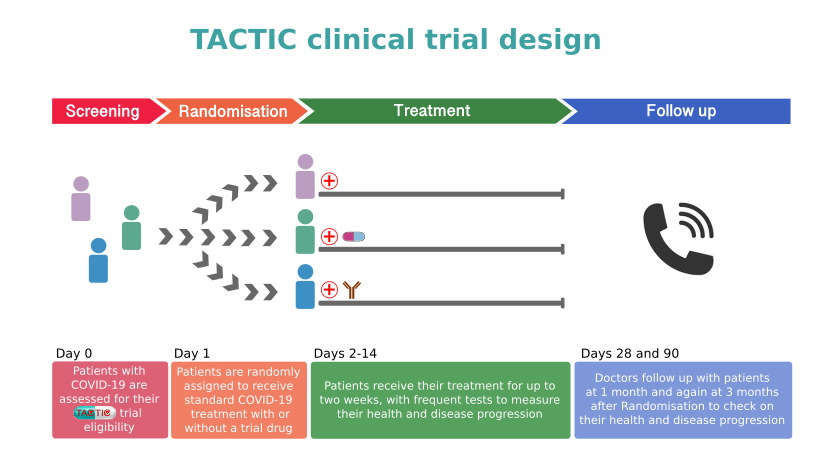
There are two trials in the TACTIC programme: TACTIC-R and TACTIC-E. TACTIC-R is testing existing drugs (known as re-purposing) which are already used to block two distinct immune response pathways. Meanwhile, TACTIC-E will look at the use of two new (experimental) treatments which are capable of modifying immune response pathways in humans such as those seen in COVID-19-related disease. The medications will be compared to a patient group receiving standard supportive care.








